Endometriosis Clinical Trial Pipeline Accelerates as 18+ Pharma Companies Rigorously Develop Drugs for Market Entry | DelveInsight
Endometriosis is a prevalent yet underdiagnosed disease affecting a large population of women of reproductive age, with a substantial and lasting impact on quality of life and fertility. While rising awareness and improved diagnostics are expanding the market, the limitations of current hormonal and surgical options are accelerating demand for novel, non-hormonal, disease-modifying therapies that offer durable relief and improved safety.
New York, USA, Jan. 22, 2026 (GLOBE NEWSWIRE) -- Endometriosis Clinical Trial Pipeline Accelerates as 18+ Pharma Companies Rigorously Develop Drugs for Market Entry | DelveInsight
Endometriosis is a prevalent yet underdiagnosed disease affecting a large population of women of reproductive age, with a substantial and lasting impact on quality of life and fertility. While rising awareness and improved diagnostics are expanding the market, the limitations of current hormonal and surgical options are accelerating demand for novel, non-hormonal, disease-modifying therapies that offer durable relief and improved safety.
DelveInsight’s 'Endometriosis Pipeline Insight 2025' report provides comprehensive global coverage of pipeline therapies for endometriosis across various stages of clinical development. The report offers an in-depth analysis of key trends, emerging therapies, and competitive landscape dynamics, highlighting the strategies of major pharmaceutical companies to advance the pipeline and capitalize on future growth opportunities. In addition, it includes critical insights into clinical trial benchmarking, partnering and licensing activities, and regulatory pathways involving the FDA and EMA, enabling stakeholders to make informed decisions and optimize development strategies within the endometriosis domain.
Endometriosis Clinical Trial Analysis Summary
- DelveInsight’s endometriosis pipeline report depicts a robust space with 18+ active players working to develop 20+ pipeline endometriosis drugs.
- Key endometriosis companies such as Hope Medicine Inc, Gesynta Pharma AB, Changchun GeneScience Pharmaceutical Co., Ltd., TiumBio, Jiangsu Hengrui Medicine Co, EpicentRx, NETRIS Pharma, Maipl Therapeutics, Temple Therapeutics, Cartherics Pty ltd, and others are evaluating new endometriosis drugs to improve the treatment landscape.
- Promising pipeline endometriosis therapies, such as HMI-115, Vipoglanstat, GenSci048, TU2670, SHR7280, Nibrozetone, NP137P, MA-4604, TTX334e, CTH-401, and others, are in different phases of endometriosis clinical trials.
- Approximately 8+ endometriosis drugs are in the mid stage of development, whereas 2+ drugs are in the early stages of development.
- Notable MoAs in endometriosis clinical trials include Prolactin receptor antagonists, Prostaglandin-E synthase inhibitors, Interleukin 1 beta inhibitors, Netrin-1 inhibitor, Gonadotropin-releasing hormone (GnRH) receptor antagonist, NLRP3 protein inhibitors, Prostaglandin F2alpha receptor antagonists, and others.
Request a sample and discover the recent advances in endometriosis drugs @ https://www.delveinsight.com/report-store/endometriosis-pipeline-insight?utm_source=globenewswire&utm_medium=pressrelease&utm_campaign=spr
What is Endometriosis?
Endometriosis involves the presence of endometrial-like glands and stromal tissue outside the uterine cavity. These ectopic lesions can manifest as peritoneal implants, superficial ovarian lesions or cysts, or as deeply infiltrating disease. While the precise etiology of endometriosis is not fully understood, several hypotheses have been advanced to explain lesion formation. One of the most commonly cited mechanisms is retrograde menstruation, documented in women and some non-human primates, in which menstrual blood flows backward through the fallopian tubes into the pelvic cavity. This process, along with potential dissemination through vascular or lymphatic pathways, may enable endometrial tissue to implant at ectopic sites.
A further challenge in the management of endometriosis is the lack of a universally accepted staging system, as standardized classification is crucial for effective communication among clinicians and for guiding treatment decisions. Since Sampson first described ovarian hematomas in 1921, numerous classification approaches have been developed. However, none has been universally adopted, largely due to the disease’s heterogeneity and the weak association between lesion extent and clinical symptoms. At present, four principal classification systems are in use: the revised American Society for Reproductive Medicine (rASRM) system, the ENZIAN classification, the Endometriosis Fertility Index (EFI), and the American Association of Gynecological Laparoscopists (AAGL) classification.
Find out more about endometriosis drugs @ Endometriosis Treatment
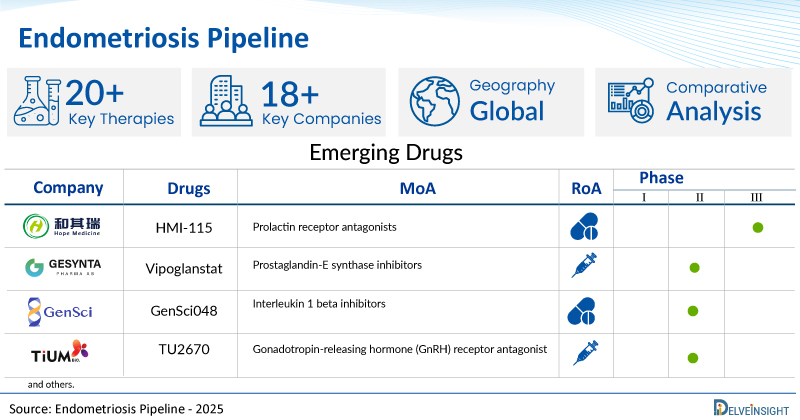
A snapshot of the Pipeline Endometriosis Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| HMI-115 | Hope Medicine Inc. | III | Prolactin receptor antagonists | Subcutaneous |
| Vipoglanstat | Gesynta Pharma AB | II | Prostaglandin-E synthase inhibitors | Oral |
| GenSci048 | Changchun GeneScience Pharmaceutical Co., Ltd. | II | Interleukin 1 beta inhibitors | Subcutaneous |
| TU2670 | TiumBio | II | Gonadotropin-releasing hormone (GnRH) receptor antagonist | Oral |
| Nibrozetone | EpicentRx | I | NLRP3 protein inhibitors | NA |
| NP137P | NETRIS Pharma | Preclinical | Netrin-1 inhibitor | NA |
| MA-4604 | Maipl Therapeutics | Preclinical | Prostaglandin F2alpha receptor antagonists | Oral |
| TTX334e | Temple Therapeutics | Preclinical | NA | NA |
Learn more about the emerging endometriosis therapies @ Endometriosis Clinical Trials
Recent Developments in Endometriosis Treatment Space
- In December 2025, Hope Medicine Inc. announced that HMI-115 received Fast Track designation from the US FDA based on data from a Phase II clinical trial for endometriosis.
- In September 2025, Gesynta Pharma AB announced that its clinical trial application for the company's Phase II trial of vipoglanstat for the treatment of endometriosis has been approved by the UK authorities. The clinical trial, named NOVA, intends to evaluate the efficacy and safety of vipoglanstat against placebo in approximately 190 patients with endometriosis across several European countries.
- In May 2025, Maipl Therapeutics, Inc. announced a strategic alliance with Endosure, Inc., the developer of the ENDOSURE TEST. The collaboration will leverage the ENDOSURE TEST to expedite Maipl's clinical studies of MA-4604, a potent and selective antagonist of the Prostaglandin‑F2α receptor (FP), with the goals of accelerating patient identification, enrollment and pharmacodynamic assessment.
- In October 2024, Hope Medicine Inc. announced positive results from an interim analysis of a global Phase II study, " A Randomized, Multicenter, Double-Blind, Placebo -Controlled Phase II Study to Evaluate the Safety and Efficacy of HMI-115 in Women with Moderate to Severe Endometriosis Associated Pain Over a 12-Week Treatment Period".
- In May 2024, NETRIS Pharma was awarded EUR 3.9m to lead the development of a Novel Therapy in Endometriosis. The program has received dedicated funding to advance its preclinical development toward clinical evaluation.
- In May 2024, TiumBio Co., Ltd. announced positive topline results from its Phase IIa clinical trial of merigolix, an oral gonadotropin-releasing hormone (GnRH) receptor antagonist, in patients with moderate to severe endometriosis-associated pain.
- In April 2024, Jiangsu Hengrui Pharmaceuticals announced that it will grant a paid license for the SHR7280 oral GnRH receptor antagonist project to Merck KGaA (German Merck), with an upfront payment of 15 million euros. In this transaction, German Merck will obtain exclusive rights for the commercialization of SHR7280 in mainland China, as well as priority negotiation rights in regions outside the licensed area.
Scope of the Endometriosis Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Intravenous, Subcutaneous, Parenteral, Topical
- Therapeutics Assessment By Molecule Type: Recombinant fusion proteins, Small molecule, Monoclonal antibody, Peptide, Polymer, Gene therapy
- Therapeutics Assessment By Mechanism of Action: Prolactin receptor antagonists, Prostaglandin-E synthase inhibitors, Interleukin 1 beta inhibitors, Netrin-1 inhibitor, Gonadotropin-releasing hormone (GnRH) receptor antagonist, NLRP3 protein inhibitors, Prostaglandin F2alpha receptor antagonists.
- Key Endometriosis Companies: Hope Medicine Inc, Gesynta Pharma AB, Changchun GeneScience Pharmaceutical Co., Ltd., TiumBio, Jiangsu Hengrui Medicine Co, EpicentRx, NETRIS Pharma, Maipl Therapeutics, Temple Therapeutics, Cartherics Pty ltd, and others.
- Key Endometriosis Pipeline Therapies: HMI-115, Vipoglanstat, GenSci048, TU2670, SHR7280, Nibrozetone, NP137P, MA-4604, TTX334e, CTH-401, and others.
Dive deep into rich insights for new endometriosis treatments, visit @ Endometriosis Drugs
Table of Contents
| 1. | Endometriosis Pipeline Report Introduction |
| 2. | Endometriosis Pipeline Report Executive Summary |
| 3. | Endometriosis Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Endometriosis Clinical Trial Therapeutics |
| 6. | Endometriosis Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Endometriosis Pipeline: Late-Stage Products (Phase III) |
| 8. | Endometriosis Pipeline: Mid-Stage Products (Phase II) |
| 9. | Endometriosis Pipeline: Early-Stage Products (Phase I) |
| 10. | Endometriosis Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Endometriosis Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Endometriosis Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the endometriosis cure research, reach out @ Medication for Endometriosis Treatment
Related Reports
Endometriosis Epidemiology Forecast
Endometriosis Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted endometriosis epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Endometriosis Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key endometriosis companies, including Mitsubishi Tanabe Pharma America, Ferring Pharmaceuticals, AbbVie, Neurocrine Biosciences, ObsEva, Kissei Pharmaceuticals, SWK, Enteris BioPharma, Bayer, Hope Medicine, Organon, among others.
Endometriosis Pain Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key endometriosis pain companies including Horizon Therapeutics (Amgen), Dompe Farmaceutici, Sylentis, MorphoSys, Resolve Therapeutics, OSE Immunotherapeutics, Servier, Novartis, Johnson & Johnson, among others.
Endometriosis Pain Clinical Trial Analysis
Endometriosis Pain Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key endometriosis pain companies, including Ferring Pharmaceuticals, ObsEva SA, Myovant Sciences, Hope Medicine (Nanjing) Co., Ltd, TiumBio Co., Ltd., Mitsubishi Tanabe Pharma, among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
